Results from Multiple Clinical Trials Demonstrate the Intracept Procedure is:
Safe
Strong safety profile with less than 0.3% rate of serious Intracept Procedure-related complications reported across nearly 400 clinical trial patients. 1
Effective
Two Level I RCTs demonstrate that the Intracept Procedure is an effective treatment compared to both a sham-control procedure and to non-surgical standard care.2,3
Durable
Significant improvements in function and pain seen at 3 months post the Intracept Procedure are sustained more than 5 years after a single treatment.2
Reproducible
Consistency across clinical studies with results reproduced in a typical community spine practice.2-6
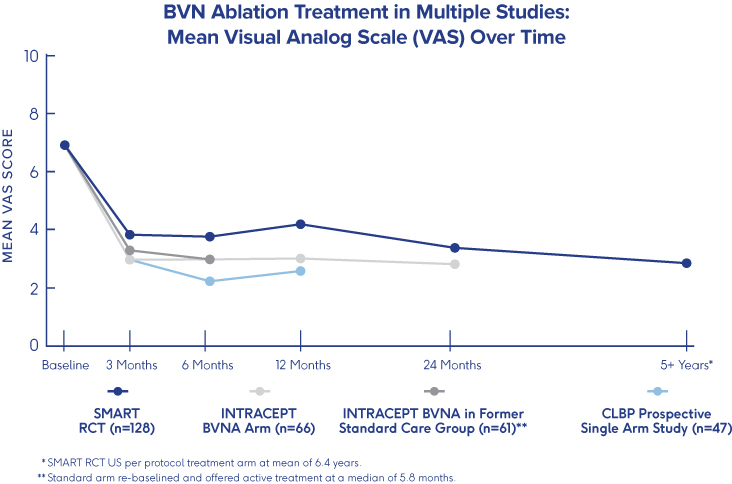
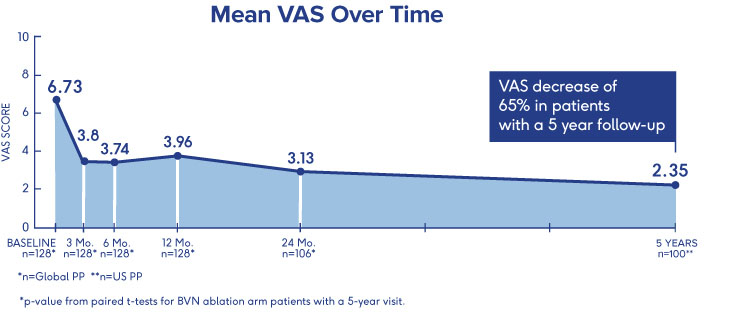
SMART Trial
5-Year Durability2
- VAS: 4.38 reduction at 5 years (from 6.74 to 2.35 (p<0.001))†
- ODI: 25.95 reduction at 5 years (from 42.81 to 16.86 (p<0.001))†
- One-third (34%) of patients were pain free at 5 years†
†p-value from paired t-tests for BVN ablation arm patients with a 5-year visit
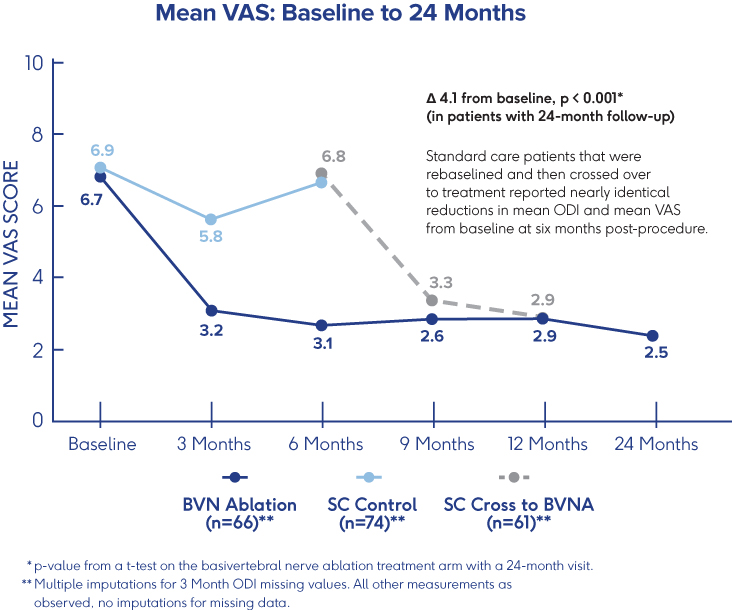
INTRACEPT Study
24-Month Results Demonstrate
Sustained Relief 3
- VAS: 4.1 cm mean reduction from baseline to 24 months post-procedure (p<0.001)†
- ODI: 28.5 point mean reduction from baseline to 24 months (p<0.001)†
- 31% of patients were pain-free 24 months after BVN ablation treatment†
†p-values from paired t-tests of BVN ablation arm patients with a 24-month visit
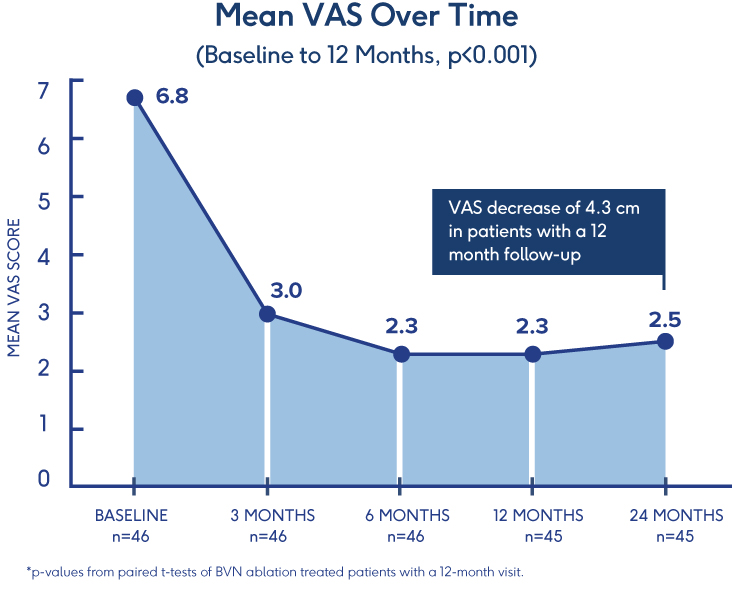
Prospective, Single-Arm Cohort Study
Sustained Relief at 12 Months in
Typical Spine Practices6
- VAS: Mean reduction of 4.3 (p<0.001) from a baseline of 6.82†
- ODI: Mean improvement of 32.31 (p<0.001) from a baseline of 46.98†
- 69% of patients reported >50% reduction in pain while 38% reported being pain-free†
†p-values from paired t-tests of BVN ablation treated patients with a 12-month visit
Pilot Clinical Study
Efficacy of BVN Ablation
in a Clinical Setting 7
- Ablation of the BVN for the treatment of chronic lumbar back pain significantly improved patients’ self-reported outcome early in the follow-up period
- Improvements in function persisted throughout the 1-year study period
- ODI: Statistically significant mean baseline reduction from 52±13 to 23±21 at 3 months follow-up (p<.001); maintained through 12-month follow-up

